Abstract
Introduction: Acute myelogenous leukemia (AML) is a major indication for allogeneic hematopoietic cell transplantation (alloHCT). Myeloablative (MAC) regimens are preferred for their intensity, however, reduced intensity conditioning regimens (RIC) have been increasingly used to permit older patients and patients with comorbidities to undergo transplant. The literature is conflicting regarding the comparison of outcomes between RIC and MAC patients. While MAC regimens are associated with less relapse, non-relapse mortality (NRM) is higher. RIC regimens on the other hand, demonstrate less NRM but higher relapse rates. We sought to compare RIC vs MAC regimens at our centre for alloHCT of AML in complete remission (CR).
Methods: We retrospectively analyzed the outcomes of 451 patients that underwent alloHCT for AML at the Princess Margaret Cancer Centre (PMCC) with either RIC or MAC using various donors between the years 2015-2020. Primary endpoint was 2-year overall survival (OS), secondary endpoints included cumulative incidence of relapse (CIR), cumulative NRM, and GVHD free/relapse free survival (GRFS).
Conditioning regimen intensity was determined as defined by Bacigalupo et al (BBMT 2009). Of the total cohort 331 patients received RIC and 121 MAC. Among MAC regimens, 59% received FLU(4)Bu(4) (Fludarabine 140mg/m2 + Busulfan 12.8mg/m2). Among RIC patients 71% received Flu(4)Bu(2)TBI(200) (Fludarabine 140mg/m2+ Busulfan 6.4 mg/m2 + TBI 200cGy).
Results:
Median age at transplant was 48 years (range: 19-67) for MAC recipients and 61 (range: 18-75) for RIC recipients (p<0.001). Other patient characteristics are described in Table 1. For the entire cohort at 2 years, the incidence of NRM was 19.1% for MAC (95% CI: 12.4-26.8) and 22.5% for RIC (95% CI: 18.1-27.1) (p=0.44). Two-year CIR was 19.8% (95% CI: 12.9-27.8) for MAC recipients and 24.5% (95% CI: 20.0-29.4) for RIC recipients (p=0.15). Two-year OS was 61% and 53% for MAC and RIC, respectively (p=0.02). Acute GVHD occurred in 58.3% of MAC recipients versus 46.8% of RIC recipients. Chronic GVHD occurred in 43.3% of the MAC recipients versus 38% of the RIC recipients. At two years GRFS was 40.8% (95% CI: 35.4-46.1) for MAC and 33.7% (95% CI: 25.0-42.6) for RIC, p=0.30. When examining the Disease Risk Index (DRI) for the entire cohort, OS for high risk was 43.3% (95% CI: 32.5-53.5) vs 66.6% (95% CI: 61-71) and 64% (95% CI: 38-80) for intermediate risk and low risk respectively, p<0.001.
A propensity score (PS) matched analysis was done matching patients for age, HLA matching (mismatched vs full match), DRI (low, intermediate, high risk) and in vivo T cell depletion (yes vs no). Two-year OS was 67% for the MAC recipients (95% CI: 57-76) and 66% for the RIC recipients (95% CI: 56-75), p=0.95 (Figure 1a). NRM for MAC was 22% (95% CI: 14-30) versus 14% (95% CI: 8-22) for RIC, p=0.19. CIR at 2 years was 18.5% for MAC (95% CI 11-27) and 24.3% for RIC (95% CI: 16-33), p=0.22. GRFS at 2 years was 36% (95% CI: 26.4-45.7) for MAC and 48.3% (95% CI: 38-58) for RIC recipients, p=0.17.
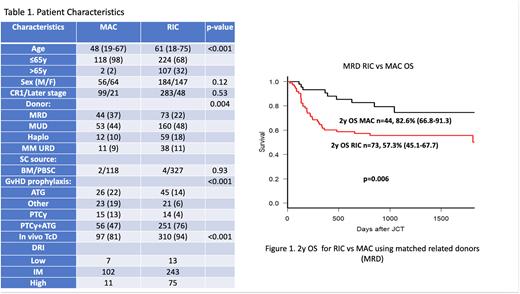
In the subgroup analysis examining matched related donor (MRD) recipients in particular, the OS at 2 years was for MAC 83.6% (95% CI: 66.8-91.3) vs 57.3% (95% CI: 45.1-67.7) for RIC, p=0.006 (Figure 1b) Two-year NRM was 10.3% for MAC vs 23.4% for the RIC group, p=0.14. CIR at 2 years was 12.2% for MAC vs 23.4% for RIC in the MRD setting, p=0.11.
Conclusions: Our study shows that in the MRD setting there is a statistically significant improved survival, especially in patients with low and intermediate DRI scores.
In other donor settings, there is no significant difference in outcomes between patients that undergo alloHCT with MAC vs RIC regimens. MAC conditioning regimens should continue to be the preferred choice of intensity for patients that may tolerate it.
Disclosures
Law:Jazz Educational: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Atara: Research Funding; Incyte Corporation: Research Funding; Sierra: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal